N95, FFP2 and KN95
Filtering face masks are subject to various regulatory standards and compliance guidelines around the world. These guidelines and standards specify the required physical properties, performance characteristics and functionality necessary for having a particular standard within a particular country. During emergency situations such as coronavirus, health authorities may authorize the use of certain foreign standards of face masks such as N95, KN95, or FFP2.
The most common face mask standards across the globe are as follows:
N95 (US NIOSH)
FFP2/FFP3 (Europe CE EN 149)
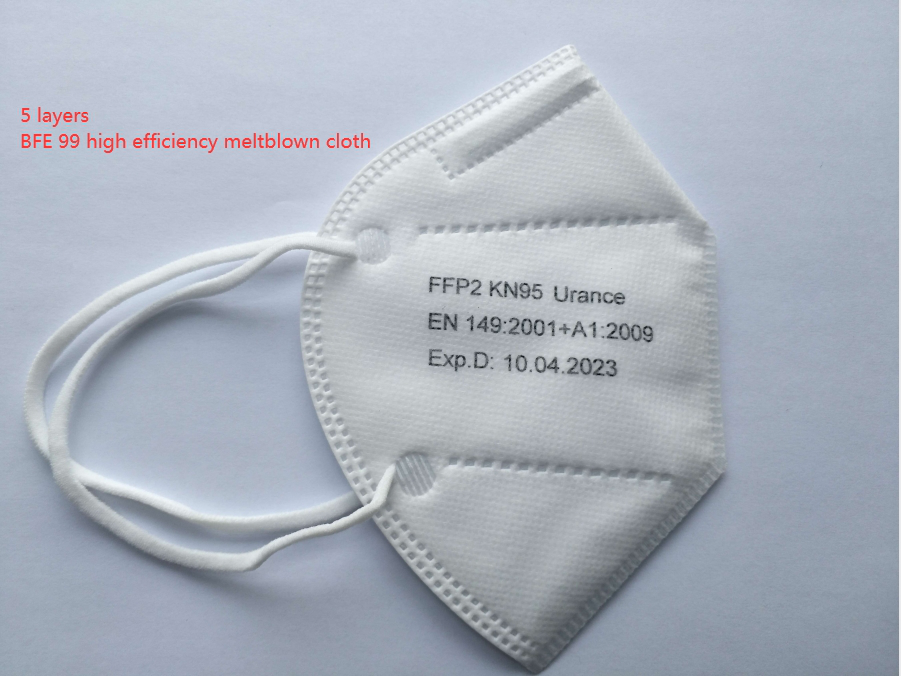
KN95(China GB2626-2006)
N95 Masks
In the US, the most commonly available and popular mask are the N95 mask which filter up to 95% of particles and create an air tight seal around the face. This type of mask is not specifically regulated by the FDA, rather they are regulated by the CDC and NIOSH. The FDA may regulate an N95 mask if the manufacturer chooses to produce the mask for a medical use, and in such case it would need an FDA clearance. However, on April 1st, 2020, in response to the coronavirus pandemic, the FDA released guidance that certain NIOSH approved respirators not currently regulated by the FDA are cleared to be used in a healthcare setting by health care personnel during the coronavirus outbreak.
FFP2 Masks
FFP2 masks are certified by the EU to meet the standards of CE regulation EN 149 for filtering up to 94% of particles and creating an air tight seal around the face. In response to the coronavirus outbreak, on March 28th, 2020, the FDA announced that certified European CE EN-149 FFP2 masks are cleared for emergency use under certain conditions when it is in lack of NIOSH approved N95 masks.
KN95 Masks
KN95 masks are regulated by the Chinese government under regulation GB2626-2006 and are rated to filter 95% of particles and create an air tight seal around the face. In April of 2020, in order to help expand the availability of general use face masks for the general public and particulate filtering mask for healthcare professionals during the coronavirus, the FDA issued guidance authorizing the use of KN95 masks as suitable NIOSH alternatives under certain emergency circumstances.
N95 Masks vs KN95 Masks vs FFP2
N95 and KN95 masks both filter up to 95% of particles and create an air tight seal around the face. Under the current US regulatory framework mentioned above, as of April 8th 2020, the FDA has authorized the emergency use of KN95 masks and FFP2 masks where there is a shortage of NIOSH approved N95 masks.
Other differences between N95 masks vs KN95 masks are that KN95 masks often use the earloop method of wearing the respirator whereas the N95 masks use a two straps attachment, one that goes around the back of the head under the ears and another that goes on the top of the head above the ears. It really comes down to personal preference in terms of which one is more comfortable for the wearer.
Personally, I find that the KN95 is easier and faster to put on and take off whereas the N95 seems to fit much more snug and with a tighter and firmer fit. Further, I find that the N95 masks, due to the tightness of the seal, are slightly more uncomfortable to wear, but give a feeling of greater protection. Another factor to consider is that NIOSH approved N95 masks tend to be a little pricier than the KN95/FFP2 variants.
So with a competitive price and big sourcing capacity of KN95 70K per day, we’d like to help with our customers around the world and hopefully we can stand together to get over this pandemic.